Dry ice is simply frozen CO2. It is inexpensive and readily available. Look under “dry ice” in the Yellow Pages. Unlike water, CO2 has no liquid stage at atmospheric pressures. It transforms directly from solid to gas as it thaws.
A pound of dry ice equals a pound of CO2. A pound of CO2 equals 8.7 cubic feet. By timing the period required for a chunk of a certain size to melt, a fairly good estimate can be made of the amount of CO2 put into the atmosphere during that time period.
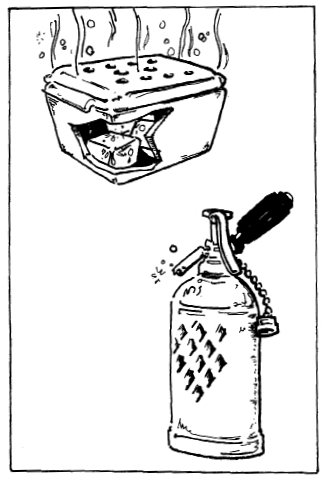
Some practitioners utilize a block of dry ice put into an insulating device such as a foam ice cooler with holes cut into the top and sides. The size and number of holes allow control of the rate at which the block melts and releases CO2. (See drawing on page 38.)
Advantages of Dry Ice
- Dry ice is inexpensive and easily obtained.
- Dry ice is non-toxic, but the extreme cold can damage tissue.
Wear insulated gloves when moving unwrapped chunks.
(Pants of some sort are also a good idea around large exposed chunks of dry ice.)
- Dry ice requires no flame, electricity, or moving parts to use.
- Dry ice is compact and more easily moved than tanks of CO2 gas.
- Dry ice does not put any heat or water into the atmosphere. The frozen CO2 actually helps to control heat due to the fact that of dry ice remains frozen below 100 degrees below zero!
Disadvantages of Dry Ice
- The continuous generation of CO2 gas limits the use of exhaust fan cycles.
- There is a period of less than optimum CO2 levels while the room is slowly filling.
- While the melting rate can be slowed by keeping dry
ice in a food freezer, it can not be stopped. It is difficult to
store dry ice at home.